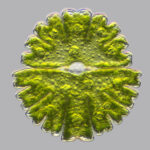
Barium (Ba) has a special use in a single group of living organisms, the single-celled Desmidiales or Desmids, green algae containing Barium Sulfate BaSO4 crystals in constant Brownian motion. It is suspected these heavy molecules, suspended in one end of the cell only, may help it sense gravity, space, and facilitate up and down orientation.
Barium, the 6th period element of the 2nd group is a heavy atom, much heavier than its upper neighbor Strontium, yet not nearly as heavy as the true heavyweights among the elements, such as Gold (79) or Lead (82). All elements heavier still (except Bismuth) are unstable, too big and in decay, slowly disintegrating and therefore radioactive.
Barium is opaque to x-rays and used daily in hospitals. People are fed a “Barium Meal” before x-ray images are taken of their intestines, showing these in a clear outline. Barium itself would be highly toxic, so again we see Barium Sulfate being used here. These ions Ba2+ and SO42- are like best friends, very hard to separate. Their atomic bond is so strong that even the acidity of the stomach does not affect it, and for the better. Barium would block Potassium moving out of nerve cells, killing nervous activities and life.
read the full text...